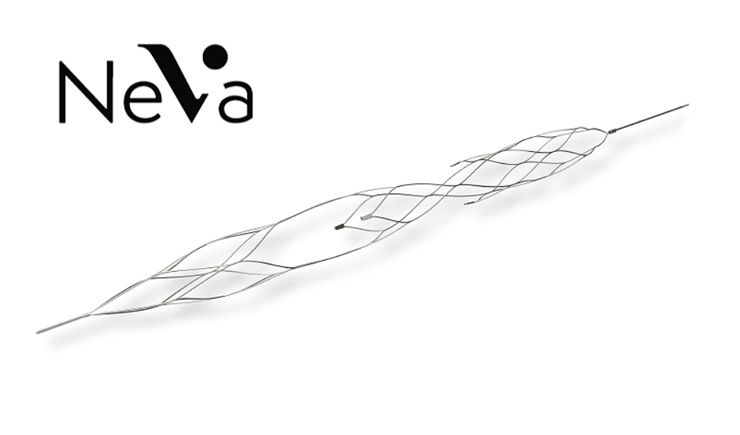
Vesalio’s NeVa Thrombectomy Device First-Pass Rates Evaluated in Published Study
Vesalio announced that data on the use of the company’s NeVa thrombectomy device were published by Arsida Bajrami, MD, et al in Interventional Neuroradiology. The company stated that the NeVa platform is designed to consistently achieve first-pass recanalization in stroke by effectively removing all types of neurovascular clots from a patient’s anatomy.
According to Vesalio, the data collected from 145 stroke patients constitutes the largest patient series to date on the technology and underscores the device’s excellent first-pass and final recanalization scores (77%, first pass; 98%, final modified Thrombolysis in Cerebral Infarction [mTICI] 2B-3). The company noted that, in Interventional Neuroradiology, the investigators concluded the NeVa, “offers a high rate of first-pass success along with a favorable safety profile.”
The company also announced the appointment of Bob Bushok as Vice President of United States Sales, overseeing commercial strategy and execution for the launch of the NeVa VS vasospasm device and the expected introduction in 2023 of the NeVa thrombectomy platform into the United States market.
Mr. Bushok will be responsible for building the company’s United States sales team and will play a pivotal role in continuing to grow the company through strategic initiatives.
He has 20 years of sales leadership experience in the neurovascular space with Medtronic and several successful start-ups, including Concentric, EV3, and Viz.AI. Most recently, Mr. Bushok served as Senior Director at Viz.AI, where he was responsible for integrated delivery networks, national accounts, and pioneering artificial intelligence use to increase the speed and access to care in acute ischemic stroke.
Source: Endovascular Today