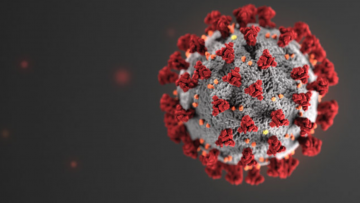
FDA REMOVES THE RED TAPE ON TEST DEVELOPERS MARCH 18
Despite a decade of tightly regulating testing, the FDA is now allowing companies to proceed with their diagnostic tests for COVID-19 without first submitting them for federal review or obtaining an official emergency clearance. In the past, the FDA has warned of the personal health risks that could follow
FDA OKs HYPERFINE’s BEDSIDE MRI
The FDA has approved 510 (k) clearance for the Hyperfine Research Inc. bedside Magnetic Resonance Imaging (MRI) system, the world’s first bedside MRI. The Hyperfine system is 20 times lower cost, 35 times lower power consumption, and 10 times less weight than today’s fixed conventional MRI systems. Jonathan Rothberg,
VICI VENOUS STENT: FDA OKs VICI Venous Stent to treat Iliofemoral obstructive disease
The U.S Food and Drug Administration has approved Boston Scientific’s VICI Venous Stent System for the treatment of iliofemoral venous obstructive disease, which occurs when the flow of blood through the veins located deep in the pelvic region becomes blocked by a blood clot or compressed by anatomical anomalies.
FDA evaluating stents, balloons with paclitaxel coating following JAHA evaluation
A December study in The Journal of the American Heart Association by Konstantinos Katsanos, MD, of Rion, Greece, et al, reported a higher risk of death after balloons and stents used to open vessels in the legs were coated with an anti-inflammatory chemo agent called paclitaxel. “Further investigations are urgently
WHEN MEDICINE MAKES PATIENTS SICKER
By Sydney Lupkin Photos by Heidi de Marco Despite the jackhammer-like rhythm of a mechanical ventilator, Alicia Moreno had dozed off in a chair by her 1-year-old’s hospital bed, when a doctor woke her with some bad news: The common stool softener her son, Anderson, was given months earlier had been
AVINGER RECEIVES FDA CLEARANCE FOR PANTHERIS DEVICE; SHARES AHEAD 47% PREMARKET
Avinger has received 510(k) clearance from the FDA for its next generation Pantheris Lumivascular atherectomy system, the first-ever image-guided atherectomy device for the treatment of peripheral artery disease (P.A.D.). Lumivascular is the only technology that combines real-time intravascular imaging with highly effective catheters for the treatment of P.A.D. The Company intends to launch two versions
SURMODICS GETS FDA OK TO SELL TELEMARK MICROCATHETHETER
The U.S. Federal Food and Drug Administration has given Surmodics clearance to sell its Telemark .014-inch coronary and peripheral support microcatheter. The 510(k) approval allows Surmodics to start shipping a device designed for use in the treatment of peripheral lesions and complex coronary and peripheral lesions. Telemark brings together
