
ABBVIE ACQUIRING ALLERGAN AS HEDGE AGAINST EXPIRATION OF HUMIRA PATENT
As a hedge against the 2023 expiration of its U.S. patents that protect its blockbuster drug, Humira, AbbVie Inc. will acquire Dublin, Ireland-based Allergan for $63 billion in cash and stock. Humira sales account for more than 60 percent of AbbVie’s revenue, but biosimilar drugs approved in Europe
RATIONAL SUICIDE: Seniors want to call it quits on own schedule
(Caitlin Hillyard/KHN) By Melissa Bailey Ten residents slipped away from their retirement community one Sunday afternoon for a covert meeting in a grocery store cafe. They aimed to answer a taboo question: When they feel they have lived long enough, how can they carry out their own swift and peaceful
VSA, PRACTICE MANAGEMENT FIRM, OBSERVES 17TH YEAR
Vein Centers of America (VSA) is celebrating its 17th anniversary by offering a complimentary phone consultation with President and CEO David Schmiege. in vein-specific practice management, marketing and revenue cycle management initiatives nationwide. “We have the experience, flexibility, structure and size to implement results quickly and to create the culture

DO YOUR OLDER PATIENTS REGARD THEIR HEALTH AS GOOD OR POOR?
By Judith Graham A common myth about aging is that older adults are burdened by illness and feel lousy much of the time. In fact, the opposite is usually true. Most seniors report feeling distinctly positive about their health. Consider data from the 2017 National Health Interview Survey (the most
VICI VENOUS STENT: FDA OKs VICI Venous Stent to treat Iliofemoral obstructive disease
The U.S Food and Drug Administration has approved Boston Scientific’s VICI Venous Stent System for the treatment of iliofemoral venous obstructive disease, which occurs when the flow of blood through the veins located deep in the pelvic region becomes blocked by a blood clot or compressed by anatomical anomalies.
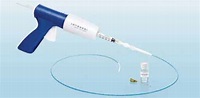
VECLOSE EXTENSION STUDY: Five-year outcomes prove durability, safety, efficacy of VenaSeal Closure System
By Larry Storer Five-year results from the 2019 VeClose Extension Study of the Medtronic VenaSeal Closure System demonstrate that the treatment modality is consistent, durable and safe for permanently closing veins in patients with venous reflux disease. When compared with Medtronic Radiofrequency Ablation, VenaSeal continued to show “non-inferiority”

MERZ REGAINS SOLE DISTRIBUTION OF ASCLERA FROM ANGIODYNAMICS
Merz North America Inc. has regained exclusive distribution of Asclera (polidocanol) Injection in the United States to all healthcare practitioners and phlebologists treating varicose veins. Also effective April 8, AngioDynamics Inc. is no longer an authorized distributor of Asclera. All HCPs will now be able to purchase Asclera directly
AVLS offers sclerotherapy and hands-on ultrasound courses Aug. 10
Margaret Mann, MD, FACPh, will conduct the American Vein and Lymphatic Society Sclerotherapy Course Aug. 10 in Arlington, Va. The Sclerotherapy Course is a comprehensive one-day lecture series, reviewing both the theoretical and practical essentials of sclerotherapy. This course will enhance your sclerotherapy knowledge

SOCIAL SECURITY ERROR JEOPARDIZES MEDICARE COVERAGE FOR 250,000 SENIORS
By Susan Jaffe At least a quarter of a million Medicare beneficiaries may receive bills for as many as five months of premiums they thought they already paid. But they shouldn’t toss the letter in the garbage. It’s not a scam or a mistake. Because of what the
A LARGE EMPLOYER ‘FRAMES’ THE ‘MEDICARE FOR ALL’ DEBATE
By Phil Galewitz Walk into a big-box retailer such as Walmart or Michaels and you’re likely to see MCS Industries’ picture frames, decorative mirrors or kitschy wall décor. Adjacent to a dairy farm a few miles west of downtown Easton, MCS is the nation’s largest maker of such household
