CAN SOMEONE TELL ME WHAT A PBM REALLY DOES?
By Rachel Bluth It was back-to-school day at the Senate Finance Committee hearing April 9. In the third of a series of hearings on rising drug prices, the senators seemed focused on getting an answer to one central question: What the heck is a pharmacy benefit manager? Pharmacy benefit managers,
Teleflex gets premarket OK for biomechanical MANTA Closure
Teleflex Inc., a global provider of medical technologies for critical care and surgery, has announced that it received premarket approval from the U.S. Food and Drug Administration for the MANTA Vascular Closure Device – the first commercially available biomechanical vascular closure device designed specifically for large bore femoral arterial
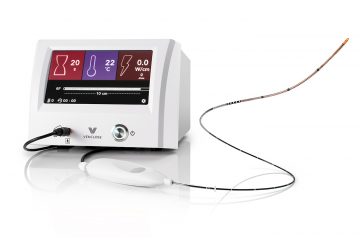
A NEW RF SYSTEM: VENCLOSE 2ND GENERATION RADIOFREQUENCY TECHNOLOGY OUTPERFORMS LEGACY SYSTEM
By Larry Storer The great medical technology companies of our time start and develop in a variety of ways. Some start with a product or idea and built a market around it. Some start as spinoffs from existing successful companies. But Venclose started by determining the unmet needs
FDA evaluating stents, balloons with paclitaxel coating following JAHA evaluation
A December study in The Journal of the American Heart Association by Konstantinos Katsanos, MD, of Rion, Greece, et al, reported a higher risk of death after balloons and stents used to open vessels in the legs were coated with an anti-inflammatory chemo agent called paclitaxel. “Further investigations are urgently
INTERVENTIONAL RADIOLOGY SOCIETIES CALL FOR EXPANDED STROKE TRAINING
The Society of Interventional Radiology (SIR), the Cardiovascular and Interventional Radiology Society of Europe and the Interventional Radiology Society of Australasia have formed a Joint Commission committed to providing necessary stroke training to interventional radiologists in order to alleviate the shortage of physicians trained in endovascular stroke therapies. The ability for patients
MDLINX SURVEY: MAJORITY HAVE LOST FAITH IN VALUE OF THE MOC
A survey of 515 U.S. physicians by MDLinx revealed that 65 percent want to see the 10-year maintenance of certification (MOC) process ended because it adds no clinical value to the practice of medicine. The recent survey, reported in FierceHealthcare, found that almost 55 percent of respondents want to see those controversial MOC requirements
MEDTRONIC GETS OK TO STUDY PATIENTS WITH BICUSPID AORTIC VALUES USING COREVALVE EVOLUT TAVR SYSTEM
The U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) for Medtronic to initiate a single-arm study to evaluate the CoreValve Evolut TAVR system in patients with bicuspid aortic valves who are at low risk of surgical mortality. Medtronic separately received FDA approval for revised commercial
IT’S A LITTLE-KNOWN SECRET THAT PATIENTS CAN GET THOUSANDS OF DOLLARS DIRECTLY FROM A DRUGMAKER
By Sarah Jane Tribble Kip Burgess was relieved last year when pharmaceutical giant Amgen overnighted him a $2,976 check to help pay for his go-to arthritis drug, Enbrel. The 36-year-old psychologist had run into an increasingly common problem: The copay coupon sent by Thousand Oaks, Calif.-based Amgen

IN.PACT ADMIRAL: MEDTRONIC SHARES FIVE-YEAR DRUG-COATED BALLOON DATA
Medtronic plc data presented Nov. 12 reinforce the durability, safety, and effectiveness of the IN.PACT Admiral drug-coated balloon (DCB) in patients with peripheral artery disease in the superficial femoral and popliteal arteries. The five-year and final results from the pivotal IN.PACT SFA Trial and one-year all-subjects results from the
KENNETH OSORIO, MD: A successful vein specialist leads missions of mercy to Caraz, Peru
By John A. Chuback, MD, FACS, RPVI, RVT, RPhS Kenneth Osorio, MD, is a successful phlebologist and the medical director of Advanced Vein Center, a very active clinical practice in Mesa, Arizona. Despite his many professional accomplishments, he has not forgotten his roots; nor has Dr. Osorio forgotten
