LETHAL PLANS: When seniors turn to suicide in long-term care
By Melissa Bailey and JoNel Aleccia When Larry Anders moved into the Bay at Burlington nursing home in late 2017, he wasn’t supposed to be there long. At 77, the stoic Wisconsin machinist had just endured the death of his wife of 51 years and a grim new diagnosis: throat

MERZ NORTH AMERICA REGAINS EXCLUSIVE DISTRIBUTION OF ASCLERA (POLIDOCANOL) INJECTION
Merz North America Inc. has regained exclusive distribution of Asclera (polidocanol) Injection in the United States to all healthcare practitioners and phlebologists treating varicose veins, effective April 8. Also effective April 8th, AngioDynamics Inc. is no longer an authorized distributor of Asclera. All healthcare professionals will now be able
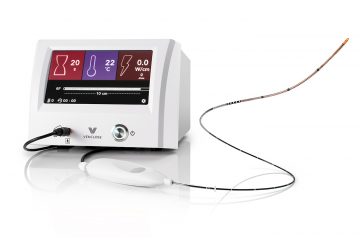
A NEW RF SYSTEM: VENCLOSE 2ND GENERATION RADIOFREQUENCY TECHNOLOGY OUTPERFORMS LEGACY SYSTEM
By Larry Storer The great medical technology companies of our time start and develop in a variety of ways. Some start with a product or idea and built a market around it. Some start as spinoffs from existing successful companies. But Venclose started by determining the unmet needs
SIGVARIS CHANGES CORPORATE NAME, LOGO
SIGVARIS has changed its corporate name from SIGVARIS to SIGVARIS GROUP and has also changed its logo. SIGVARIS has a 150-year history of proven success, according to CEO Mark Bunker. “We are the world leader in medical compression socks and hosiery with a wide assortment of
Virtual reality immerses physicians in AVLS education offerings
By Ron Seybold The American Vein and Lymphatic Society is launching a new project to use immersive virtual reality (VR) technology for its educational courses. The educational an array of venous medicine techniques. VR has started to make it possible to gain a better view than ever of fundamental and cutting-edge
Covidien kickback scheme draws $17.4 million fine
Covidien LP, the developer and promoter of radiofrequency vein ablation, has agreed to pay $17,477,947 to resolve allegations that it violated the False Claims Act by providing free or discounted practice development and market development support to physicians located in California and Florida to induce purchases of Covidien’s vein ablation
FDA evaluating stents, balloons with paclitaxel coating following JAHA evaluation
A December study in The Journal of the American Heart Association by Konstantinos Katsanos, MD, of Rion, Greece, et al, reported a higher risk of death after balloons and stents used to open vessels in the legs were coated with an anti-inflammatory chemo agent called paclitaxel. “Further investigations are urgently
INTERVENTIONAL RADIOLOGY SOCIETIES CALL FOR EXPANDED STROKE TRAINING
The Society of Interventional Radiology (SIR), the Cardiovascular and Interventional Radiology Society of Europe and the Interventional Radiology Society of Australasia have formed a Joint Commission committed to providing necessary stroke training to interventional radiologists in order to alleviate the shortage of physicians trained in endovascular stroke therapies. The ability for patients
MDLINX SURVEY: MAJORITY HAVE LOST FAITH IN VALUE OF THE MOC
A survey of 515 U.S. physicians by MDLinx revealed that 65 percent want to see the 10-year maintenance of certification (MOC) process ended because it adds no clinical value to the practice of medicine. The recent survey, reported in FierceHealthcare, found that almost 55 percent of respondents want to see those controversial MOC requirements
MEDTRONIC GETS OK TO STUDY PATIENTS WITH BICUSPID AORTIC VALUES USING COREVALVE EVOLUT TAVR SYSTEM
The U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) for Medtronic to initiate a single-arm study to evaluate the CoreValve Evolut TAVR system in patients with bicuspid aortic valves who are at low risk of surgical mortality. Medtronic separately received FDA approval for revised commercial
